WHAT WE DO
pharma company in bhiwadi pharma company in bhiwadi pharma company in bhiwadi pharma company in bhiwadi pharma company in bhiwadi pharma company in bhiwadi pharma company in bhiwadi pharma company in bhiwadi pharma company in bhiwadi pharma company in bhiwadi pharma company in bhiwadi pharma company in bhiwadi pharma company in bhiwadi pharma company in bhiwadi pharma company in bhiwadi pharma company in bhiwadi pharma company in bhiwadi pharma company in bhiwadi pharma company in bhiwadi pharma company in bhiwadi pharma company in bhiwadi pharma company in bhiwadi pharma company in bhiwadi pharma company in bhiwadi pharma company in bhiwadi pharma company in bhiwadi pharma company in bhiwadi pharma company in bhiwadi pharma company in bhiwadi pharma company in bhiwadi pharma company in bhiwadi pharma company in bhiwadi pharma company in bhiwadi pharma company in bhiwadi pharma company in bhiwadi pharma company in bhiwadi pharma company in bhiwadi pharma company in bhiwadi pharma company in bhiwadi pharma company in bhiwadi pharma company in bhiwadi pharma company in bhiwadi pharma company in bhiwadi pharma company in bhiwadi pharma company in bhiwadi pharma company in bhiwadi pharma company in bhiwadi pharma company in bhiwadi pharma company in bhiwadi pharma company in bhiwadi pharma company in bhiwadi pharma company in bhiwadi pharma company in bhiwadi pharma company in bhiwadi pharma company in bhiwadi pharma company in bhiwadi pharma company in bhiwadi pharma company in bhiwadi pharma company in bhiwadi pharma company in bhiwadi pharma company in bhiwadi pharma company in bhiwadi pharma company in bhiwadi pharma company in bhiwadi pharma company in bhiwadi pharma company in bhiwadi pharma company in bhiwadi pharma company in bhiwadi pharma company in bhiwadi pharma company in bhiwadi
Kusum’s quality medicine & pharmaceutical formulations are in accordance with the stringent requirements of GMP (Good Manufacturing Practice) that are incorporated right from the production stage. A team of highly qualified staff constantly monitors all production procedures. The company’s confidence in the quality of its pharmaceutical products are based on its strict control over the drug manufacturing processes for every stage.Kusum’s quality medicine & pharmaceutical formulations are in accordance with the stringent requirements of GMP (Good Manufacturing Practice) that are incorporated right from the production stage. A team of highly qualified staff constantly monitors all production procedures. The company’s confidence in the quality of its pharmaceutical products are based on its strict control over the drug manufacturing processes for every stage.Kusum’s quality medicine & pharmaceutical formulations are in accordance with the stringent requirements of GMP (Good Manufacturing Practice) that are incorporated right from the production stage. A team of highly qualified staff constantly monitors all production procedures. The company’s confidence in the quality of its pharmaceutical products are based on its strict control over the drug manufacturing processes for every stage.Kusum’s quality medicine & pharmaceutical formulations are in accordance with the stringent requirements of GMP (Good Manufacturing Practice) that are incorporated right from the production stage. A team of highly qualified staff constantly monitors all production procedures. The company’s confidence in the quality of its pharmaceutical products are based on its strict control over the drug manufacturing processes for every stage.Kusum’s quality medicine & pharmaceutical formulations are in accordance with the stringent requirements of GMP (Good Manufacturing Practice) that are incorporated right from the production stage. A team of highly qualified staff constantly monitors all production procedures. The company’s confidence in the quality of its pharmaceutical products are based on its strict control over the drug manufacturing processes for every stage.Kusum’s quality medicine & pharmaceutical formulations are in accordance with the stringent requirements of GMP (Good Manufacturing Practice) that are incorporated right from the production stage. A team of highly qualified staff constantly monitors all production procedures. The company’s confidence in the quality of its pharmaceutical products are based on its strict control over the drug manufacturing processes for every stage.Kusum’s quality medicine & pharmaceutical formulations are in accordance with the stringent requirements of GMP (Good Manufacturing Practice) that are incorporated right from the production stage. A team of highly qualified staff constantly monitors all production procedures. The company’s confidence in the quality of its pharmaceutical products are based on its strict control over the drug manufacturing processes for every stage.Kusum’s quality medicine & pharmaceutical formulations are in accordance with the stringent requirements of GMP (Good Manufacturing Practice) that are incorporated right from the production stage. A team of highly qualified staff constantly monitors all production procedures. The company’s confidence in the quality of its pharmaceutical products are based on its strict control over the drug manufacturing processes for every stage.Kusum’s quality medicine & pharmaceutical formulations are in accordance with the stringent requirements of GMP (Good Manufacturing Practice) that are incorporated right from the production stage. A team of highly qualified staff constantly monitors all production procedures. The company’s confidence in the quality of its pharmaceutical products are based on its strict control over the drug manufacturing processes for every stage.Kusum’s quality medicine & pharmaceutical formulations are in accordance with the stringent requirements of GMP (Good Manufacturing Practice) that are incorporated right from the production stage. A team of highly qualified staff constantly monitors all production procedures. The company’s confidence in the quality of its pharmaceutical products are based on its strict control over the drug manufacturing processes for every stage.Kusum’s quality medicine & pharmaceutical formulations are in accordance with the stringent requirements of GMP (Good Manufacturing Practice) that are incorporated right from the production stage. A team of highly qualified staff constantly monitors all production procedures. The company’s confidence in the quality of its pharmaceutical products are based on its strict control over the drug manufacturing processes for every stage.Kusum’s quality medicine & pharmaceutical formulations are in accordance with the stringent requirements of GMP (Good Manufacturing Practice) that are incorporated right from the production stage. A team of highly qualified staff constantly monitors all production procedures. The company’s confidence in the quality of its pharmaceutical products are based on its strict control over the drug manufacturing processes for every stage.Kusum’s quality medicine & pharmaceutical formulations are in accordance with the stringent requirements of GMP (Good Manufacturing Practice) that are incorporated right from the production stage. A team of highly qualified staff constantly monitors all production procedures. The company’s confidence in the quality of its pharmaceutical products are based on its strict control over the drug manufacturing processes for every stage.Kusum’s quality medicine & pharmaceutical formulations are in accordance with the stringent requirements of GMP (Good Manufacturing Practice) that are incorporated right from the production stage. A team of highly qualified staff constantly monitors all production procedures. The company’s confidence in the quality of its pharmaceutical products are based on its strict control over the drug manufacturing processes for every stage.Kusum’s quality medicine & pharmaceutical formulations are in accordance with the stringent requirements of GMP (Good Manufacturing Practice) that are incorporated right from the production stage. A team of highly qualified staff constantly monitors all production procedures. The company’s confidence in the quality of its pharmaceutical products are based on its strict control over the drug manufacturing processes for every stage.Kusum’s quality medicine & pharmaceutical formulations are in accordance with the stringent requirements of GMP (Good Manufacturing Practice) that are incorporated right from the production stage. A team of highly qualified staff constantly monitors all production procedures. The company’s confidence in the quality of its pharmaceutical products are based on its strict control over the drug manufacturing processes for every stage.Kusum’s quality medicine & pharmaceutical formulations are in accordance with the stringent requirements of GMP (Good Manufacturing Practice) that are incorporated right from the production stage. A team of highly qualified staff constantly monitors all production procedures. The company’s confidence in the quality of its pharmaceutical products are based on its strict control over the drug manufacturing processes for every stage.Kusum’s quality medicine & pharmaceutical formulations are in accordance with the stringent requirements of GMP (Good Manufacturing Practice) that are incorporated right from the production stage. A team of highly qualified staff constantly monitors all production procedures. The company’s confidence in the quality of its pharmaceutical products are based on its strict control over the drug manufacturing processes for every stage.Kusum’s quality medicine & pharmaceutical formulations are in accordance with the stringent requirements of GMP (Good Manufacturing Practice) that are incorporated right from the production stage. A team of highly qualified staff constantly monitors all production procedures. The company’s confidence in the quality of its pharmaceutical products are based on its strict control over the drug manufacturing processes for every stage.Kusum’s quality medicine & pharmaceutical formulations are in accordance with the stringent requirements of GMP (Good Manufacturing Practice) that are incorporated right from the production stage. A team of highly qualified staff constantly monitors all production procedures. The company’s confidence in the quality of its pharmaceutical products are based on its strict control over the drug manufacturing processes for every stage.Kusum’s quality medicine & pharmaceutical formulations are in accordance with the stringent requirements of GMP (Good Manufacturing Practice) that are incorporated right from the production stage. A team of highly qualified staff constantly monitors all production procedures. The company’s confidence in the quality of its pharmaceutical products are based on its strict control over the drug manufacturing processes for every stage.Kusum’s quality medicine & pharmaceutical formulations are in accordance with the stringent requirements of GMP (Good Manufacturing Practice) that are incorporated right from the production stage. A team of highly qualified staff constantly monitors all production procedures. The company’s confidence in the quality of its pharmaceutical products are based on its strict control over the drug manufacturing processes for every stage.Kusum’s quality medicine & pharmaceutical formulations are in accordance with the stringent requirements of GMP (Good Manufacturing Practice) that are incorporated right from the production stage. A team of highly qualified staff constantly monitors all production procedures. The company’s confidence in the quality of its pharmaceutical products are based on its strict control over the drug manufacturing processes for every stage.Kusum’s quality medicine & pharmaceutical formulations are in accordance with the stringent requirements of GMP (Good Manufacturing Practice) that are incorporated right from the production stage. A team of highly qualified staff constantly monitors all production procedures. The company’s confidence in the quality of its pharmaceutical products are based on its strict control over the drug manufacturing processes for every stage.Kusum’s quality medicine & pharmaceutical formulations are in accordance with the stringent requirements of GMP (Good Manufacturing Practice) that are incorporated right from the production stage. A team of highly qualified staff constantly monitors all production procedures. The company’s confidence in the quality of its pharmaceutical products are based on its strict control over the drug manufacturing processes for every stage.Kusum’s quality medicine & pharmaceutical formulations are in accordance with the stringent requirements of GMP (Good Manufacturing Practice) that are incorporated right from the production stage. A team of highly qualified staff constantly monitors all production procedures. The company’s confidence in the quality of its pharmaceutical products are based on its strict control over the drug manufacturing processes for every stage.Kusum’s quality medicine & pharmaceutical formulations are in accordance with the stringent requirements of GMP (Good Manufacturing Practice) that are incorporated right from the production stage. A team of highly qualified staff constantly monitors all production procedures. The company’s confidence in the quality of its pharmaceutical products are based on its strict control over the drug manufacturing processes for every stage.Kusum’s quality medicine & pharmaceutical formulations are in accordance with the stringent requirements of GMP (Good Manufacturing Practice) that are incorporated right from the production stage. A team of highly qualified staff constantly monitors all production procedures. The company’s confidence in the quality of its pharmaceutical products are based on its strict control over the drug manufacturing processes for every stage.Kusum’s quality medicine & pharmaceutical formulations are in accordance with the stringent requirements of GMP (Good Manufacturing Practice) that are incorporated right from the production stage. A team of highly qualified staff constantly monitors all production procedures. The company’s confidence in the quality of its pharmaceutical products are based on its strict control over the drug manufacturing processes for every stage.Kusum’s quality medicine & pharmaceutical formulations are in accordance with the stringent requirements of GMP (Good Manufacturing Practice) that are incorporated right from the production stage. A team of highly qualified staff constantly monitors all production procedures. The company’s confidence in the quality of its pharmaceutical products are based on its strict control over the drug manufacturing processes for every stage.Kusum’s quality medicine & pharmaceutical formulations are in accordance with the stringent requirements of GMP (Good Manufacturing Practice) that are incorporated right from the production stage. A team of highly qualified staff constantly monitors all production procedures. The company’s confidence in the quality of its pharmaceutical products are based on its strict control over the drug manufacturing processes for every stage.Kusum’s quality medicine & pharmaceutical formulations are in accordance with the stringent requirements of GMP (Good Manufacturing Practice) that are incorporated right from the production stage. A team of highly qualified staff constantly monitors all production procedures. The company’s confidence in the quality of its pharmaceutical products are based on its strict control over the drug manufacturing processes for every stage.Kusum’s quality medicine & pharmaceutical formulations are in accordance with the stringent requirements of GMP (Good Manufacturing Practice) that are incorporated right from the production stage. A team of highly qualified staff constantly monitors all production procedures. The company’s confidence in the quality of its pharmaceutical products are based on its strict control over the drug manufacturing processes for every stage.Kusum’s quality medicine & pharmaceutical formulations are in accordance with the stringent requirements of GMP (Good Manufacturing Practice) that are incorporated right from the production stage. A team of highly qualified staff constantly monitors all production procedures. The company’s confidence in the quality of its pharmaceutical products are based on its strict control over the drug manufacturing processes for every stage.Kusum’s quality medicine & pharmaceutical formulations are in accordance with the stringent requirements of GMP (Good Manufacturing Practice) that are incorporated right from the production stage. A team of highly qualified staff constantly monitors all production procedures. The company’s confidence in the quality of its pharmaceutical products are based on its strict control over the drug manufacturing processes for every stage.Kusum’s quality medicine & pharmaceutical formulations are in accordance with the stringent requirements of GMP (Good Manufacturing Practice) that are incorporated right from the production stage. A team of highly qualified staff constantly monitors all production procedures. The company’s confidence in the quality of its pharmaceutical products are based on its strict control over the drug manufacturing processes for every stage.Kusum’s quality medicine & pharmaceutical formulations are in accordance with the stringent requirements of GMP (Good Manufacturing Practice) that are incorporated right from the production stage. A team of highly qualified staff constantly monitors all production procedures. The company’s confidence in the quality of its pharmaceutical products are based on its strict control over the drug manufacturing processes for every stage.Kusum’s quality medicine & pharmaceutical formulations are in accordance with the stringent requirements of GMP (Good Manufacturing Practice) that are incorporated right from the production stage. A team of highly qualified staff constantly monitors all production procedures. The company’s confidence in the quality of its pharmaceutical products are based on its strict control over the drug manufacturing processes for every stage.Kusum’s quality medicine & pharmaceutical formulations are in accordance with the stringent requirements of GMP (Good Manufacturing Practice) that are incorporated right from the production stage. A team of highly qualified staff constantly monitors all production procedures. The company’s confidence in the quality of its pharmaceutical products are based on its strict control over the drug manufacturing processes for every stage.Kusum’s quality medicine & pharmaceutical formulations are in accordance with the stringent requirements of GMP (Good Manufacturing Practice) that are incorporated right from the production stage. A team of highly qualified staff constantly monitors all production procedures. The company’s confidence in the quality of its pharmaceutical products are based on its strict control over the drug manufacturing processes for every stage.Kusum’s quality medicine & pharmaceutical formulations are in accordance with the stringent requirements of GMP (Good Manufacturing Practice) that are incorporated right from the production stage. A team of highly qualified staff constantly monitors all production procedures. The company’s confidence in the quality of its pharmaceutical products are based on its strict control over the drug manufacturing processes for every stage.Kusum’s quality medicine & pharmaceutical formulations are in accordance with the stringent requirements of GMP (Good Manufacturing Practice) that are incorporated right from the production stage. A team of highly qualified staff constantly monitors all production procedures. The company’s confidence in the quality of its pharmaceutical products are based on its strict control over the drug manufacturing processes for every stage.Kusum’s quality medicine & pharmaceutical formulations are in accordance with the stringent requirements of GMP (Good Manufacturing Practice) that are incorporated right from the production stage. A team of highly qualified staff constantly monitors all production procedures. The company’s confidence in the quality of its pharmaceutical products are based on its strict control over the drug manufacturing processes for every stage.Kusum’s quality medicine & pharmaceutical formulations are in accordance with the stringent requirements of GMP (Good Manufacturing Practice) that are incorporated right from the production stage. A team of highly qualified staff constantly monitors all production procedures. The company’s confidence in the quality of its pharmaceutical products are based on its strict control over the drug manufacturing processes for every stage.Kusum’s quality medicine & pharmaceutical formulations are in accordance with the stringent requirements of GMP (Good Manufacturing Practice) that are incorporated right from the production stage. A team of highly qualified staff constantly monitors all production procedures. The company’s confidence in the quality of its pharmaceutical products are based on its strict control over the drug manufacturing processes for every stage.Kusum’s quality medicine & pharmaceutical formulations are in accordance with the stringent requirements of GMP (Good Manufacturing Practice) that are incorporated right from the production stage. A team of highly qualified staff constantly monitors all production procedures. The company’s confidence in the quality of its pharmaceutical products are based on its strict control over the drug manufacturing processes for every stage.Kusum’s quality medicine & pharmaceutical formulations are in accordance with the stringent requirements of GMP (Good Manufacturing Practice) that are incorporated right from the production stage. A team of highly qualified staff constantly monitors all production procedures. The company’s confidence in the quality of its pharmaceutical products are based on its strict control over the drug manufacturing processes for every stage.Kusum’s quality medicine & pharmaceutical formulations are in accordance with the stringent requirements of GMP (Good Manufacturing Practice) that are incorporated right from the production stage. A team of highly qualified staff constantly monitors all production procedures. The company’s confidence in the quality of its pharmaceutical products are based on its strict control over the drug manufacturing processes for every stage.Kusum’s quality medicine & pharmaceutical formulations are in accordance with the stringent requirements of GMP (Good Manufacturing Practice) that are incorporated right from the production stage. A team of highly qualified staff constantly monitors all production procedures. The company’s confidence in the quality of its pharmaceutical products are based on its strict control over the drug manufacturing processes for every stage.Kusum’s quality medicine & pharmaceutical formulations are in accordance with the stringent requirements of GMP (Good Manufacturing Practice) that are incorporated right from the production stage. A team of highly qualified staff constantly monitors all production procedures. The company’s confidence in the quality of its pharmaceutical products are based on its strict control over the drug manufacturing processes for every stage.Kusum’s quality medicine & pharmaceutical formulations are in accordance with the stringent requirements of GMP (Good Manufacturing Practice) that are incorporated right from the production stage. A team of highly qualified staff constantly monitors all production procedures. The company’s confidence in the quality of its pharmaceutical products are based on its strict control over the drug manufacturing processes for every stage.Kusum’s quality medicine & pharmaceutical formulations are in accordance with the stringent requirements of GMP (Good Manufacturing Practice) that are incorporated right from the production stage. A team of highly qualified staff constantly monitors all production procedures. The company’s confidence in the quality of its pharmaceutical products are based on its strict control over the drug manufacturing processes for every stage.Kusum’s quality medicine & pharmaceutical formulations are in accordance with the stringent requirements of GMP (Good Manufacturing Practice) that are incorporated right from the production stage. A team of highly qualified staff constantly monitors all production procedures. The company’s confidence in the quality of its pharmaceutical products are based on its strict control over the drug manufacturing processes for every stage.Kusum’s quality medicine & pharmaceutical formulations are in accordance with the stringent requirements of GMP (Good Manufacturing Practice) that are incorporated right from the production stage. A team of highly qualified staff constantly monitors all production procedures. The company’s confidence in the quality of its pharmaceutical products are based on its strict control over the drug manufacturing processes for every stage.Kusum’s quality medicine & pharmaceutical formulations are in accordance with the stringent requirements of GMP (Good Manufacturing Practice) that are incorporated right from the production stage. A team of highly qualified staff constantly monitors all production procedures. The company’s confidence in the quality of its pharmaceutical products are based on its strict control over the drug manufacturing processes for every stage.Kusum’s quality medicine & pharmaceutical formulations are in accordance with the stringent requirements of GMP (Good Manufacturing Practice) that are incorporated right from the production stage. A team of highly qualified staff constantly monitors all production procedures. The company’s confidence in the quality of its pharmaceutical products are based on its strict control over the drug manufacturing processes for every stage.Kusum’s quality medicine & pharmaceutical formulations are in accordance with the stringent requirements of GMP (Good Manufacturing Practice) that are incorporated right from the production stage. A team of highly qualified staff constantly monitors all production procedures. The company’s confidence in the quality of its pharmaceutical products are based on its strict control over the drug manufacturing processes for every stage.Kusum’s quality medicine & pharmaceutical formulations are in accordance with the stringent requirements of GMP (Good Manufacturing Practice) that are incorporated right from the production stage. A team of highly qualified staff constantly monitors all production procedures. The company’s confidence in the quality of its pharmaceutical products are based on its strict control over the drug manufacturing processes for every stage.Kusum’s quality medicine & pharmaceutical formulations are in accordance with the stringent requirements of GMP (Good Manufacturing Practice) that are incorporated right from the production stage. A team of highly qualified staff constantly monitors all production procedures. The company’s confidence in the quality of its pharmaceutical products are based on its strict control over the drug manufacturing processes for every stage.Kusum’s quality medicine & pharmaceutical formulations are in accordance with the stringent requirements of GMP (Good Manufacturing Practice) that are incorporated right from the production stage. A team of highly qualified staff constantly monitors all production procedures. The company’s confidence in the quality of its pharmaceutical products are based on its strict control over the drug manufacturing processes for every stage.
Kusum’s quality medicine & pharmaceutical formulations are in accordance with the stringent requirements of GMP (Good Manufacturing Practice) that are incorporated right from the production stage. A team of highly qualified staff constantly monitors all production procedures. The company’s confidence in the quality of its pharmaceutical products are based on its strict control over the drug manufacturing processes for every stage.
In the past 5 years, Kusum’s pharmaceutical manufacturing facilities have passed more than 60 government & medical body audits from around the world. Kusum firmly believes that “Human life and health are invaluable, and there cannot be any compromise.”
Formulations & Development
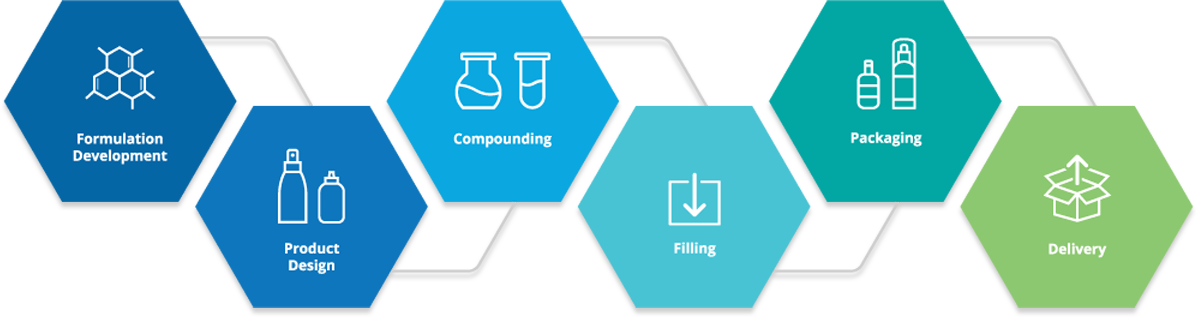
Global Manufacturing
Kusum runs world-class pharmaceutical manufacturing plants at Bhiwadi, Rajasthan and Pithampur, Indore, MP. The pharmaceutical units and systems are designed to meet & exceed all international norms. Its ISO 9001 and ISO 14001 certified plants manufacture pharmaceutical oral solids and topical dosage forms such as tablets, pellets, topical liquids, creams, ointments, shampoo and wax-based suppositories and pessaries. Pharmaceutical packaging facilities are available in the form of blisters, cold forming blisters, sachets, bottles, and tubes. CGMP & Good Documentation Practices resulted in the consistent quality of pharmaceutical products. All core manufacturing areas have dedicated heating, ventilating, and air-conditioning (HVAC) systems with 15 Air Handling Units (AHUs) and High-Efficiency Particulate Air (HEPA) filters to ensure air purity within the production area.

The pharmaceutical production premises are equipped with PLC-based equipment, which meets CGMP standards. From raw material inputs, packaging material, and in-process checks, strict guidelines are institutionalized to prevent contamination. Access to the production area is restricted, and utmost care is taken to keep the area germ-free. Our obligation to manufacture quality pharmaceutical products ensures that there are no shortcuts.
In-house R&D

Kusum’s pharmaceutical R&D (Research & Development) Facility is equipped with world-class pharmaceutical infrastructure and an industry-leading workforce developing various solid dosage forms along with the capability to handle semi-solid and liquid dosage forms.
We have a specialized team that spearheads pharmaceutical research in the development of market-specific products. In addition, our development team possesses substantial experience and expertise in formulation & development and transfer of pharmaceutical technology. Our key focus areas are Immediate Release, Modified Release & Sustained Release Formulations.
Our drug formulation development team undertakes work on new active substances and generics and further improves effectiveness of existing pharmaceutical products.
All formulations are developed in-house at the state-of-art pharmaceutical formulation R&D center, recognized by the Ministry of Science and Technology, Government of India. The company currently has over 100 dossiers available, with a strong regulatory team to provide dossiers in CTD and ACTD formats to commercialize new formulations.
